This website contains information about
INBRIJA® (levodopa inhalation powder)
M-INB-UK-0047
Date of preparation: December 2025
This website is intended for healthcare professionals from the United Kingdom only. Adverse event reporting information can be found at the bottom of the page.


For OFF episodes in Parkinson’s Disease
INBRIJA® is indicated for the intermittent treatment of episodic motor fluctuations (OFF episodes) in adult patients with Parkinson’s disease treated with a levodopa/dopa-decarboxylase inhibitor.1
Inhaled levodopa, on-demand:
Supporting patients when needed,
up to 5 times per day1,2
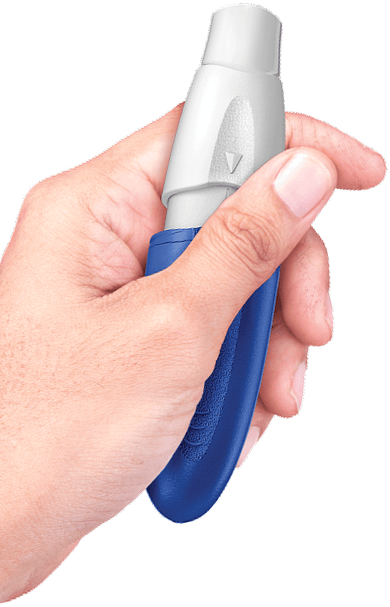
Observed relief in as little as 10 minutes2,3
Pulmonary route avoids variability caused by GI tract absorption4
Supporting patients when needed, up to 5 times per day1,4
GI; Gastrointestinal.
M-INB-UK-0047
Date of preparation: December 2025